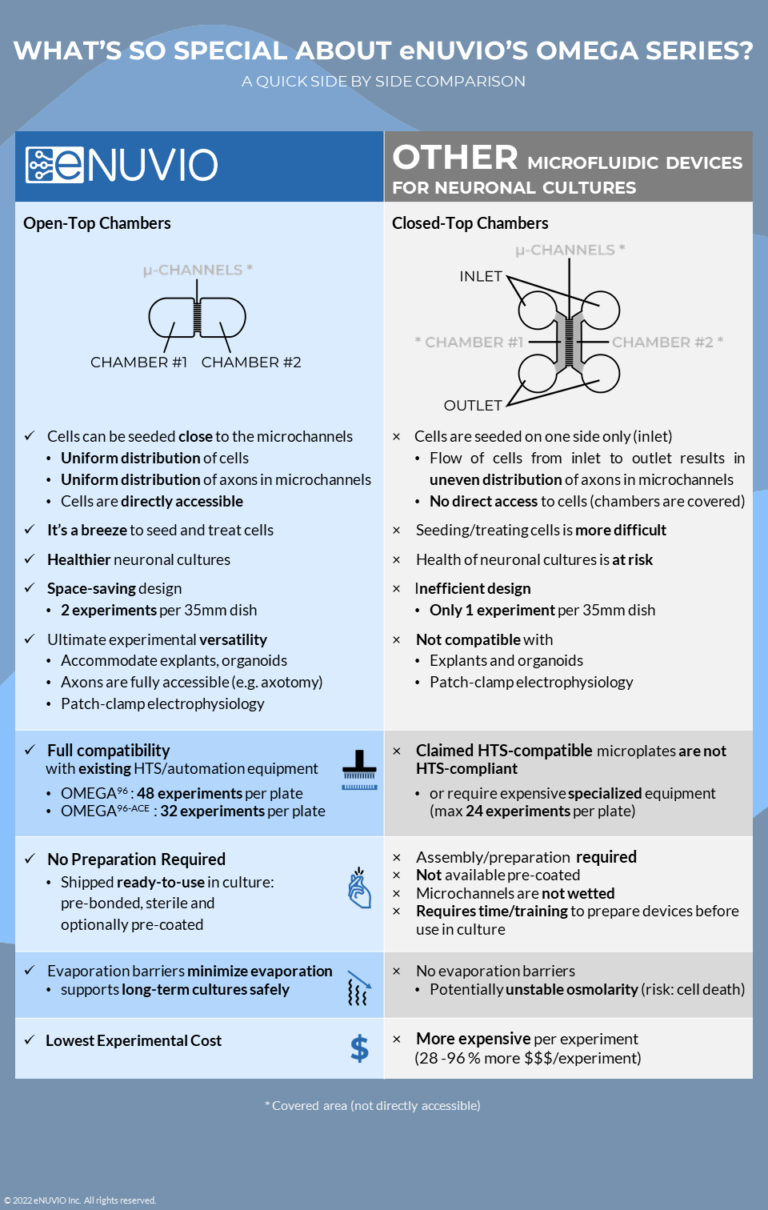
The Future of Neuronal Co-Culture Devices
In this article, we cover the advantages of microfluidic devices over standard culture vessels, some of the shortcomings of conventional microfluidic devices, and then explain how a new design can address these challenges. If you’re already familiar with microfluidic neuronal cell culture devices, you can simply skim this article and check out our side by side comparison infographic on new “open-top” versus conventional “closed-top” microfluidic neuronal culture devices for a concise overview.
Microfluidic Devices Help Recreate Important Aspects of Neuronal Physiology
Physiological relevance is one of the key characteristics to developing insightful and useful cell-based disease models. Generally, induced pluripotent stem cell (iPSC)-derived neuronal cell cultures recapitulate many important characteristics of neurons at the level of their expression profile, basic cellular morphology, and functionality. However, it is important to recognize that neurons are highly polarized cells, and that this polarity is the basis for their main electrophysiological function as well as the formation of highly organized and compartmentalized structures in vivo that are critical for proper physiological function.
When neurons are seeded in standard laboratory culture vessels, they will project neurites (dendrites and axons) in random directions. The resulting neuronal “rat’s nest” is simply not representative of the morphology and organization of neurons observed in vivo. As a way to address this, neuroscientists have turned to utilizing microfluidic and microfabricated technologies to replace the shortcomings of simple culture vessels such as standard well microplates, petri dishes or T-flasks.
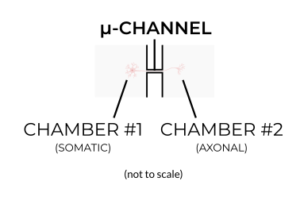
In their simplest application, these microfluidic devices replicate and enhance neuronal structural polarity in vitro by employing micron-sized channels, which physically separate two adjacent seeding chambers.
Despite their small size, these microchannels are large enough for dendrites and axons to pass through and project into the adjacent chamber, but are too small for the cell soma to pass. In this way, the microchannels effectively segregate or compartmentalize the neurons into somatic and axonal components, mimicking the natural organization of neurons observed in vivo.
Just think about motor neurons for a second. The somas of lower motor neurons are located in the ventral horn of the spinal cord where they project axons that go on to form the neuromuscular junction with skeletal muscle located some distance away in the periphery (in some cases this distance can be as much as 1 meter). Recapitulating this in a microfluidic neuronal culture device, motor neurons seeded in one chamber project their axons through the microchannels into an adjacent chamber containing skeletal muscle. In this case, the motor neuron soma is never in direct contact with the skeletal muscle culture, just like in vivo.
Conventional Microfluidic Culture Devices
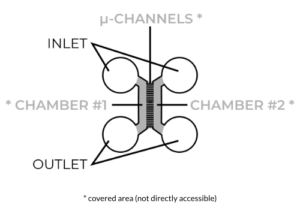 Fabricating microfluidic devices is challenging. Without going into detail, the standard microfabrication method used to make conventional neuronal compartmentalization and co-culture devices have resulted in devices having a butterfly shape or design that many conventional devices have in common.
Fabricating microfluidic devices is challenging. Without going into detail, the standard microfabrication method used to make conventional neuronal compartmentalization and co-culture devices have resulted in devices having a butterfly shape or design that many conventional devices have in common.
Unfortunately, this design has several limitations when it comes to applying them to neuronal cultures. One of the major drawbacks is its “closed-top” or covered design of the culture areas located directly adjacent to either side of the microchannels. The close-top design means that cells cannot be added to the area directly adjacent to the microchannels (where we need them to be), and instead must be unilaterally “flowed” into this area through a punched inlet well. This method of unilaterally flowing cells leads to an uneven distribution of cells in the chambers, and can result in an uneven distribution of axons within microchannels (read: increasing variability of the experiment).
There is the additional problem of applying this close-top design to standard automated or robotic liquid handling systems for high-throughput screening (HTS) applications. The unilateral flow required to seed closed-top devices cannot be readily performed by most automated liquid handling systems. Sure, you could remove half the pipette tips from each box, avoid using peristaltic pump-based 8-, 12- and 96-channel robotic liquid handlers, and cut your throughput by more than half, but all this clearly defeats the purpose of utilizing automated systems to scale up throughput in the first place!
Since the cells aren’t directly accessible (they are covered), there is a great limitation to the types of cultures and experiments that can be performed with these closed-top microfluidic devices. It becomes practically impossible (short of destroying the devices) to perform electrophysiology experiments that depend on physical access to the cultures. Importantly, you also wouldn’t use larger tissue explants or 3D cultures (sometimes measuring several millimeters in size) with these devices since they would need to be placed several millimeters away from the microchannels.
A New Design for Ultimate Versatility
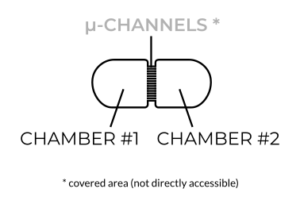 For improved versatility, a better design is needed. This new design should facilitate uniform plating of cells and provide direct access to the culture chambers so that at least 3D cultures and tissue explants can be used. In recent years, eNUVIO came up with a streamlined design, which features open, directly accessible chambers.
For improved versatility, a better design is needed. This new design should facilitate uniform plating of cells and provide direct access to the culture chambers so that at least 3D cultures and tissue explants can be used. In recent years, eNUVIO came up with a streamlined design, which features open, directly accessible chambers.
With these new “open-top” devices, cells are seeded directly next to the microchannels resulting in a more uniform distribution of cells and axons in the device. The open-top design also helps to maintain healthier culture conditions for your neurons. Also, axotomy experiments, for example, can be performed more easily by simply dragging a pipette tip along the exit of the microchannels instead of attempting to use pressurized flow or chemical alternatives required by closed-top chambers. And, you guessed it, you can of course use 3D cultures and tissue explants.
The Streamlined Design Has Even More Advantages
In a basic compartmentalization and co-culture setup, this new open-top design simply features two fully accessible chambers, rather than two covered chambers each linked to an inlet and outlet well (i.e. the “butterfly” closed-top design). Due to this simplicity, you can fit more experiments per area and – and this is really next-level – also arrange them in an array that is fully automation compatible for high-throughput screening (HTS) applications. This means that the streamlined open-top design is compatible with all standard automated liquid handlers and pipetting robots, without the need for specialized add-ons or equipment (or removing half your pipette tips from your boxes!).
It doesn’t end there. Using the open-top design, even fully HTS-compatible triple chamber co-culture devices are now possible as well as other medium-throughput devices with smaller chamber sizes. The latter devices are interesting for anybody working with expensive cell lines and needs to maximize data output. More advanced devices (eg., to model 3D neuromuscular junctions) have already been developed and fully HTS-compatible versions of these will be available in the future.
Two more advantages that you should know about: First, these new devices come completely ready-to-go. So you don’t need to assemble or bond anything, or risk breaking the device before you can even start the experiments. Wouldn’t you rather save the time and focus on the experiment? Second, you would think that with all these advantages, the new generation of devices should be more expensive than conventional devices. But, the new fabrication procedure is surprisingly cost-effective and offers the lowest price per experiment compared to conventional microfluidic devices for neuronal cell cultures.
There you have it. A comprehensive comparison of conventional “butterfly” closed-top and new streamlined open-chamber microfluidic cell culture devices for neurons. What do you think? Which aspect of the new design do you like best? And what would you love to see next? Let us know. 😊
Save the image at the top of the page for an easy side-by-side comparison and have it ready for your next project planning meeting.
About eNUVIO
eNUVIO creates cutting-edge lab-on-a-chip technologies for life science research. Co-founded in 2016 by Hugo McGuire (CEO; PhD physics), Mark Aurousseau (CSO; PhD pharmacology), and Élise Faure (COO; PhD physiology), eNUVIO operates at the interface of physics, engineering and life science and is committed to providing researchers with next-generation technologies to facilitate and accelerate scientific discovery. eNUVIO’s ready-to-go and customized products are designed to provide physiological relevance to experimentation, increase data output, reduce waste and cater to the needs and workflows of life science researchers. eNUVIO has developed a variety of first-in-class sophisticated microfabricated devices for medium- to high-throughput drug screening applications that include a series of neuronal compartmentalization as well as 3D cell culture devices. All their products are designed, fabricated and manufactured in-house at their headquarters in Montreal, Canada.
LinkedIn, Instagram, Facebook, Twitter: @enuvio.
Contact:
Email: [email protected]